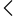RCMC under the Medical Devices Export Promotion Council is an official registration connecting medical device exporters with the Government of India trade framework. RCMC, or Registration cum Membership Certificate, is issued to exporters holding a valid Import Export Code and confirms that they are formally registered with the council recognized by the Directorate General of Foreign Trade. It serves as proof that the exporter operates within the approved medical device categories under India’s Foreign Trade Policy.
This registration is essential for claiming export incentives, duty remission benefits, and other policy-based financial support provided by DGFT. It also allows exporters to participate in council-led trade promotions, international exhibitions, and buyer-seller meetings, providing industry-specific exposure and growth opportunities.
Medical Devices EPC represents manufacturers and exporters of medical equipment, diagnostics, disposables, implants, and healthcare technology products. RCMC is provided digitally via the DGFT portal after a verification process. Once approved, exporters can download it for compliance purposes, customs procedures, and claiming government incentives. The certificate is valid for a specific period and must be renewed on time to maintain eligibility for all benefits.































































-Limited.webp)












-Private-Limited.webp)






-Private-Limited.webp)





















Pvt.Ltd.webp)








-Private-Limited.webp)