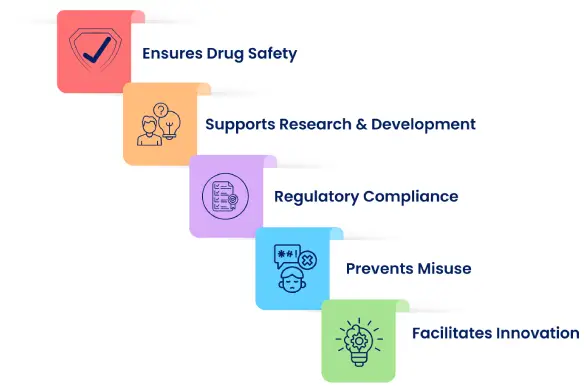
The CDSCO test license is issued under the Drugs and Cosmetics Act, 1940, and the Rules, 1945, to authorize the import or manufacture of limited quantities of drugs solely for testing, analysis, or research purposes. The CDSCO Test License ensures that every medicine or product is safe and meets the required quality standards before it reaches the market. This license is given in Form-11 by the Central Drugs Standard Control Organization (CDSCO), which works under the Ministry of Health and Family Welfare.
This rule helps keep strong control over the quality of drugs tested in labs or used in clinical studies. It is an important requirement for all research centers, factories, and testing labs that work on developing or checking new medicines. The license ensures that every product tested in India follows the country's safety rules and meets the highest standards before it is used by people. It does not allow commercial sale or marketing of imported substances.
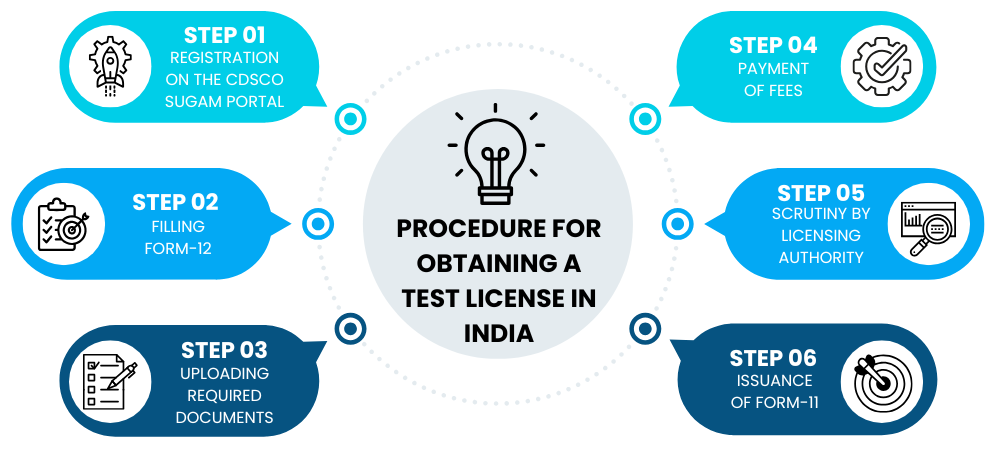
This rule helps the CDSCO ensure that all testing work is honest, safe, and in compliance with India's medicine laws. Any company, importer, or research lab that wants to test drugs must first get permission through the CDSCO SUGAM Portal. Only after getting this approval can they handle or test any drug samples. This process keeps drug research fair, safe, and fully under government control. The entire process promotes transparency, accountability, and high-quality pharmaceutical research practices in India.
What is the CDSCO SUGAM Portal?
The CDSCO SUGAM Portal is an online regulatory platform launched by the Central Drugs Standard Control Organization. The CDSCO SUGAM Portal makes it easy to apply for different types of drug approvals like test licenses, manufacturing permissions, and import approvals. It works as a single online platform where everything can be done in one place.
People can upload documents, pay the required fees, and check the progress of their applications anytime. This online system helps in faster work, clear communication, and smooth coordination between companies and the CDSCO officers. All CDSCO test license applications are handled through this portal in an organized and timely manner, ensuring that every step complies with India's drug safety and regulatory rules.































































-Limited.webp)












-Private-Limited.webp)






-Private-Limited.webp)





















Pvt.Ltd.webp)








-Private-Limited.webp)