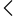India has strict rules to keep people safe and make sure medical devices work properly. Not all devices need a license right away. Some devices are non-notified because they have low risk or are still being checked for full regulation. For these devices, CDSCO allows voluntary registration to ensure they are safe and meet the required standards.
Non-notified medical device registration aims to bring each product into a proper compliance structure. This makes sure devices follow quality and safety rules before they enter the Indian healthcare market. CDSCO handles all applications through the online SUGAM portal. Local manufacturers and importers create their accounts and share basic information about their business and medical devices.
This registration makes it clear how a medical device works, what materials are used, and which safety standards are followed during production. Voluntary registration also prepares companies for the future, so they can meet stricter rules if the authorities officially require their device category.
The Medical Devices Rules 2017 support this compliance system by defining responsibilities for both manufacturers and importers. CDSCO experts review all records, permissions, certifications, and declarations before issuing the registration number. This process creates a strong control system for safe technology and better patient care across India. It also improves the global reputation of the Indian medical device industry.
What Are Non-Notified Medical Devices as per Medical Devices Rules 2017?
Non-notified medical devices are devices not listed under CDSCO's mandatory licensing categories under the Medical Devices Rules 2017. These devices do not require a formal manufacturing or import license at the initial stage. Their risk is usually lower when compared with notified devices. They still play an important role in diagnosis, monitoring, or treatment.
To ensure safety, CDSCO allows voluntary registration so every product meets strong quality standards like ISO certification. This process also prepares companies for future regulations because many device categories gradually become notified over time. Non-notified devices gain legal recognition and market acceptance through this registration. It creates confidence for doctors, hospitals, and patients in India.































































-Limited.webp)












-Private-Limited.webp)






-Private-Limited.webp)





















Pvt.Ltd.webp)








-Private-Limited.webp)