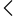India imports many medical devices, and making sure they are safe is very important. Because of this, the Central Drugs Standard Control Organisation requires importers to submit Form MD-26 before bringing any regulated device into the country. This form includes key details such as product specifications, manufacturer information, device safety records, and a Free Sale Certificate from the exporting country. It helps CDSCO check that the device meets quality standards and is safe for use in India.
In this guide, you will understand what Form MD-26 includes, who needs to apply for it, what documents are required, and how the entire import license process works. Imported medical devices directly affect hospitals, clinics, patients, and healthcare outcomes, which is why proper compliance is crucial. By clearly understanding MD-26 requirements, businesses can import devices easily, avoid regulatory problems, and make sure only safe and approved products enter the Indian market.
What Is CDSCO MD-26?
CDSCO MD-26 is the official application form used to get an import license for medical devices in India. It is issued under the Medical Device Rules, 2017, and must be submitted by any importer or authorized agent who wants to bring medical devices from a foreign manufacturer into the country. The form collects essential details about the device, including its category, purpose, safety profile, and global regulatory status. This information helps CDSCO understand the product and how it will be used in the healthcare system.
MD-26 forms the basis for determining if a medical device is suitable for the Indian market. It allows CDSCO to check the product's compliance with quality, safety, and performance standards before giving permission to import it. Once approved, the license ensures that only verified and regulated medical devices can legally enter and be distributed throughout India.































































-Limited.webp)












-Private-Limited.webp)






-Private-Limited.webp)





















Pvt.Ltd.webp)








-Private-Limited.webp)