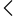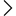FDA Approval in India refers to the authorization issued by the State Food and Drug Administration under the Drugs and Cosmetics Act, 1940. This approval permits businesses to manufacture, import, distribute, or sell drugs and cosmetics in structured categories. It serves as a legal authorization that ensures your facility and goods comply with all the necessary safety and quality standards.
Unlike the U.S. FDA, India’s FDA functions at the state level, and the rules apply on the basis of where the business operates. When a company applies for FDA Registration or an FDA License, the authority assesses equipment, infrastructure, hygiene, technical staff, quality control systems, and documentation practices. The purpose is to ensure that the business constantly maintains standards allied with national regulatory rules.
An approved facility is granted an official FDA Certificate, which serves as proof of compliance. Without this certificate, the business cannot function legally in the pharmaceutical or cosmetics sectors. Whether you are manufacturing tablets, creams, or importing finished formulations, getting FDA Certification ensures that your operations stay reliable, compliant, and ready for market distribution. Overall, FDA Approval plays an important role in protecting consumers and increasing business reliability.































































-Limited.webp)












-Private-Limited.webp)






-Private-Limited.webp)





















Pvt.Ltd.webp)








-Private-Limited.webp)